Foundation year: 1946
NACE classifier: Manufacture of pharmaceutical preparations
EU Good Manufacturing Practice certificates for finished dosage forms and active pharmaceutical ingredients
EU Good Manufactiring Certificate for Pharmaceutical Wholesaler
Russian Certificate of good Manufacturing Practice for finished dosage forms
ISO 9001 Quality Management System Certificate
ISO 14001 Environmental Mangement Systeme Certificate
ISO 50001 Energy Management Certificate
Successful PMDA and US FDA
Saudi Food and Drug Administration Certificate
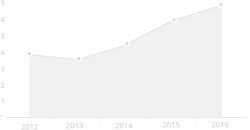
Turnover in the last 5 years
Millions EUR
Source: Data collected by the Association of Latvian Chemical and Pharmaceutical Entrepreneurs from 2018 to 2022.
“Grindeks” is an international pharmaceutical company focused on research, development, manufacturing and sales of original products, generics and active pharmaceutical ingredients. The Group of “Grindeks” has four subsidiary companies in Latvia, Estonia and Slovakia as well as representative offices in 12 countries.
“Grindeks” specializes in the heart and cardiovascular, CNS, anti-cancer and gastroenterological medication therapeutic groups. A range of products covers a combination of original products Mildronate® (meldonium*) and Ftorafur®, generics, food supplements and active pharmaceutical ingredients.
In 2017 products of the company were exported to 77 countries with export comprising 92.5% of the total turnover.
Main markets
- 1. Japan
- 2. Kazakhstan
- 3. Russia
- 4. Ukraine
Target Markets
- 1. Australia
- 2. Canada
- 3. Japan
- 4. United States
- 5. Vietnam
In which areas are looking for cooperation
Pharmaceutical products
Manufacturing of active pharmaceutical ingredients and pharmaceuticals
Products
Are you a healthcare specialist?
Showing similar companies
By the industry in which it operates
Foundation year: 1946
NACE classifier: Manufacture of pharmaceutical preparations
Turnover in the last 5 years
Millions EUR
Source: Data collected by the Association of Latvian Chemical and Pharmaceutical Entrepreneurs from 2018 to 2022.
| 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|
| 99706194 | 106774637 | 97558330 | 139312517 | 165723494 |
In which areas are looking for cooperation
Pharmaceutical products
Manufacturing of active pharmaceutical ingredients and pharmaceuticals
Export volume over the last 5 years
Data are not able
Data are not able
Main markets
- 1. Japan
- 2. Kazakhstan
- 3. Russia
- 4. Ukraine
Target Markets
- 1. Australia
- 2. Canada
- 3. Japan
- 4. United States
- 5. Vietnam
Investing in R&D
Data are not able
Data are not able

GRINDEKS AS
Description about enterprise:
“Grindeks” is an international pharmaceutical company focused on research, development, manufacturing and sales of original products, generics and active pharmaceutical ingredients. The Group of “Grindeks” has four subsidiary companies in Latvia, Estonia and Slovakia as well as representative offices in 12 countries.
“Grindeks” specializes in the heart and cardiovascular, CNS, anti-cancer and gastroenterological medication therapeutic groups. A range of products covers a combination of original products Mildronate® (meldonium*) and Ftorafur®, generics, food supplements and active pharmaceutical ingredients.
In 2017 products of the company were exported to 77 countries with export comprising 92.5% of the total turnover.
Valid certificates:
EU Good Manufacturing Practice certificates for finished dosage forms and active pharmaceutical ingredients
EU Good Manufactiring Certificate for Pharmaceutical Wholesaler
Russian Certificate of good Manufacturing Practice for finished dosage forms
ISO 9001 Quality Management System Certificate
ISO 14001 Environmental Mangement Systeme Certificate
ISO 50001 Energy Management Certificate
Successful PMDA and US FDA
Saudi Food and Drug Administration Certificate
Products
Are you a healthcare specialist?



